 |
| http://thescienceclassroom.org/chemistry-lessons/gases/the-gas-laws/ |
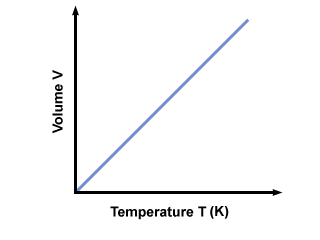
Charles Law lecture was a very short, simple lecture. It was similar to Boyle's law, so understanding the relationship of the values was easy to understand. In this law, pressure and moles are constant, leaving initial volume over initial temperature is equal to final volume over final temperature. Volume and temperature have a direct relationship, so if one increases, as does the other.
 |
| http://www.kentchemistry.com/links/GasLaws/charles.htm |


