Boyle's Law
Charles' Law
Avogadro's Law
Pressure Unit Conversions
Ideal Gas Law
Kinetic Theory of Gases
Kyle G's Pre AP Chemistry Blog
Sunday, May 8, 2016
Gas Laws Weekly Quiz
The Gas Laws quiz was not extremely difficult, but it wasn't super easy either. There were a few questions that stumped me because I did not fully understand the Kinetic Theory of energy, and the application questions were kinda hard to understand. The math questions were easy, as you basically had to just plug the numbers into the formula, and the graph questions were easy as well. I think I did okay on the quiz, and I know that I have to prepare a little bit better for the next assessment.
Charles Law
 |
| http://thescienceclassroom.org/chemistry-lessons/gases/the-gas-laws/ |
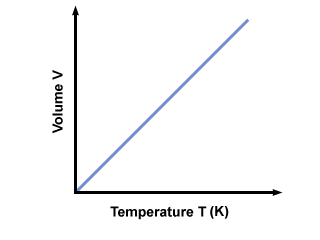 |
| http://www.kentchemistry.com/links/GasLaws/charles.htm |
Boyle's Law
 |
| http://thescienceclassroom.org/chemistry-lessons/gases/the-gas-laws/ |
| https://en.wikipedia.org/wiki/Boyle%27s_law |
Thursday, April 28, 2016
Energy and Phase Changes Unit
| http://g.web.umkc.edu/gounevt/Weblec212Silb/L3(12.3-12.4).pdf |
| http://www.kmacgill.com/lecture_notes/lecture_notes_14.htm |
| https://www.boundless.com/physics/textbooks/boundless-physics-textbook/temperature-and-kinetic-theory-12/phase-changes-106/phase-changes-and-energy-conservation-384-6949/ |
The energy and phase changes unit was a fairly simple one. As the unit was only one week long, we ran through the lessons and labs quickly, which was kind of difficult going through all of the information so fast. Although the information was pretty easy, it was difficult to memorize all of the information so fast, but it turned out okay. I prepared a lot for the test the night before, which was good for me, as I got a 95% on the test. Overall, this unit was a success, so I am happy.
 |
| http://www.mrzimmerman.org/New%20Folder/HW/STUDY%20GUIDE%20for%20Prop%20of%20Matter.htm |
Subscribe to:
Comments (Atom)